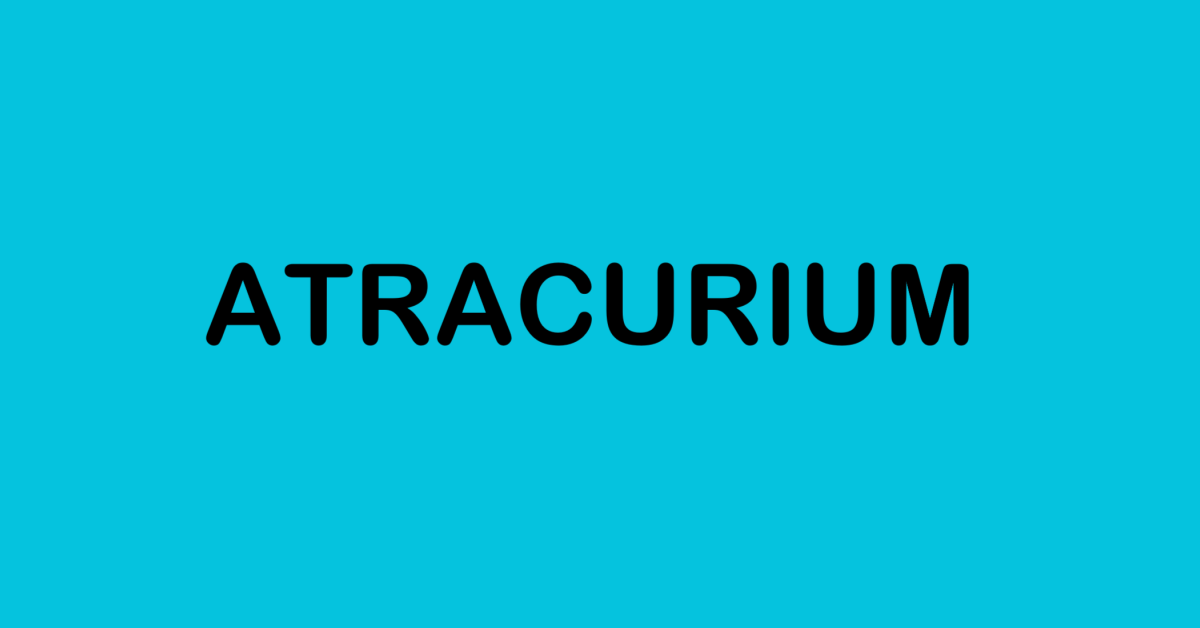
Atracurium besylate
Generic name
Atracurium besylate
Trade name
TRACRIUM®
Classification
NEUROMUSCULAR BLOCKER NON-DEPOLARISING
Indication
To facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
Dosage form
25 mg in 2.5 mL, 50mg in 5mL/ampoule
Dose
Adult :
Initial dose: 300 to 600 mcg/kg.
Supplemental doses: 50 to 200 mcg/kg/dose.
Infusion range: 300 to 600 mcg/kg/hour
Child :
Initial dose: 500 mcg/kg.
Infusion range: 300 to 600 mcg/kg/hour.
Dose adjustment
Administration
IV: Bolus or Continuous infusion
Intravenous Concentration
Bolus: 10 mg per mL ,Continuous Infusion: 50 mg in 100 mL (500 mcg per mL)
Intravenous Rate
Bolus: Inject over 1 minute , Continuous Infusion: 0.6 to 1.2 mL/kg/hour (300 to 600 mcg/kg/hour)
Storage
เก็บอุณหภูมิ 2-8 C, ป้องกันแสง, ห้ามแช่แข็ง
Reconstitution
Not required
Stability
Discard any unused solution
Compatible fluid
NSS, D5W, Ringer’S
Incompatible fluid
Incompatible drug
aminophylline, amylobarbitone, cephazolin, diazepam, heparin, phenobarbitone, phenytoin, propofol, ranitidine, sodium nitroprusside, sodium bicarbonate, thiopentone
Adverse effect
Histamine release (large doses), flushing of the skin, erythema, pruritis, urticaria, hypotension, tachycardia, bronchospasm, bradycardia (ให้ร่วม fentanyl anaesthesia)
Precaution
ห้ามให้ทาง IM
Contraindication
Information